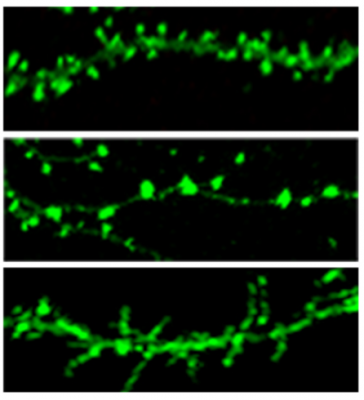
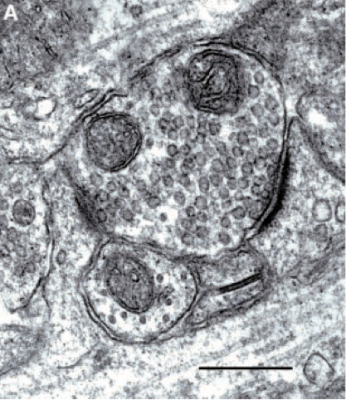
Our research is focused on the identification and characterization of proteins found at the postsynaptic density (PSD), a structure at the postsynaptic membrane of excitatory glutamatergic synapses at the tips of dendritic spines. The PSD contains proteins that organize the glutamate receptors, transduce signals initiated by receptors, promote adhesion between the postsynaptic and presynaptic membranes, and modify the synapse in response to neurological activity. Modification of synapses and dendritic spines by the proteins in the PSD is central to learning and memory and higher-order thought processes. We identified proteins in the PSD fraction with an analytical method based on mass spectrometry coupled to automated searches of protein databases. One of the newly identified synaptic proteins was IQSEC2/BRAG1, a protein that activates the Arf family of small GTPases. Arfs regulate processes such as exocytosis, endocytosis, vesicular trafficking, and cytoskeletal structure. Subsequent studies have demonstrated that mutations in IQSEC2 interfere with its activation of Arfs and cause severe intellectual disability, which indicates an important role for IQSEC2 and Arfs in cognitive processes. We also study the synaptic role of hepatocyte growth factor and its receptor, Met, in modifying synapses in response to synaptic activity. Mutations that decrease expression of Met have been linked to autism. We continue to investigate the role of these and other PSD proteins in regulating synaptic structure and function.
Selected References
Brown JC, Petersen A, Zhong L, Himelright ML, Murphy JA, Walikonis RS, Gerges NZ. (2016) Bidirectional regulation of synaptic transmission by BRAG1/IQSEC2 and its requirement in long-term depression. Nature Communications. 7:11080
Kalscheuer VM, James VM, Himelright ML, Long P, Oegema R, Jensen C, Bienek M, Hu H, Haas SA, Topf M, Hoogeboom AJ, Harvey K, Walikonis R, Harvey RJ. (2015) Novel Missense Mutation A789V in IQSEC2 Underlies X-Linked Intellectual Disability in the MRX78 Family. Frontiers in Molecular Neuroscience. 8:85.
Shoubridge S, Tarpey P, Abidi F, Ramsden SL, Rujirabanjerd S, Murphy JA, Boyle J, Shaw M, Gardner A, Proos A, Puusepp H, Raymond FL, Schwartz CE, Stevenson RE, Turner G, Field M, Walikonis RS, Harvey RJ, Hackett A, Futreal PA, Stratton MR, Gécz J. (2010) Mutations in IQSEC2, a guanine nucleotide exchange factor for Arf GTPases, causes non-syndromic intellectual disability. Nature Genetics. 42:486. PMID:20473311
Murphy J, Jensen O, Walikonis R. (2006) BRAG1, a Sec7 domain-containing protein, is a component of the postsynaptic density of excitatory synapses. Brain Research. 1120:35-45. PMID:17045249
Lim CS and Walikonis RS. (2008) Hepatocyte growth factor and c-Met promote dendrite maturation during hippocampal neuron differentiation via the Akt pathway. Cell Signaling. 20:825-835. PMCID:PMC2302788
Tyndall S, Patel S, Walikonis RS. (2007) Hepatocyte growth factor-induced enhancement of dendritic branching is blocked by inhibitors of N-methyl-D-aspartate receptors and calcium/calmodulin-dependent kinases. Journal Neuroscience Res. 85:2343-51.
Tyndall S, Walikonis R. (2007) Signaling by hepatocyte growth factor in neurons is induced by pharmacological stimulation of synaptic activity. Synapse: 61:199-204.
Tyndall S, Walikonis R. (2006) The receptor tyrosine kinase Met and its ligand hepatocyte growth factor are clustered at excitatory synapses and can enhance clustering of synaptic proteins. Cell Cycle. 5(14):1560-1568.
Contact Us
| E-mail: | randall.walikonis@uconn.edu |
|---|